The Dynamics of C-termini of Microtubules in Dendrites: A Possible Clue for the Role of Neural Cytoskeleton in the Functioning of the Brain
Jack Tuszynski, Ph.D.
page 4 of 5
Another structure I want to bring to your attention is the protrusion called c-termini. These tails are composed of about twelve to sixteen amino acids and we believe that they are actually essential to the health of neurons. They interact with a lot of things, including kinesins and MAPs[1] and other proteins as is shown in the microscopic rendition of the surface of the microtubules. They are negatively charged and have their own polarity so the two ends are different, functionally and physically. As I mentioned, c-termini are dynamic.
We are examining a hidden part of the brain where a lot of things are happening that are important for our mental cognitive functions. This simulation shows how these MAPs may rearrange themselves in the process of acquiring experience. Image 4 shows how c-termini and MAPs actually connect the microtubules with the c-termini.
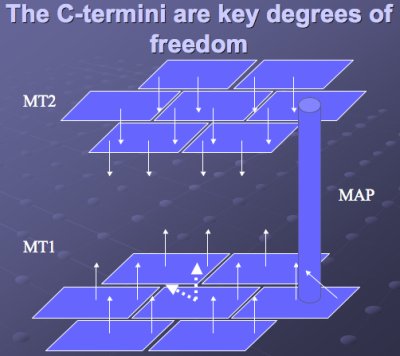
Image 4: MAPs Connecting Microtubules
Next, we looked more closely at c-termini by simulating it with simplified models. Recently, we completed a major computational analysis with atomic detail. We discovered existing c-termini in at least two other states -- functional or computational. One state is up, straight up. This is a natural state because they are negatively charged and they want to get away from the surface. We found positive charges on the surface of microtubules. They can actually bend over and bind to the positive charges and create the second state, which is a down state. This is the first component in the computational capability.
Microtubules also have two states indirectly -- a high energy state and a low energy state -- driven by the energy-giving molecules, guanosine triphosphate (GTP). Some of you may have heard of ATP, which is a basic unit of biochemical energy in living systems. GTP is its analog. Microtubules eat GTP. Motor proteins, such as kinesis, eat ATP.
Thus we have two states in terms of energy, two states in terms of the c-termini, and then possible different electronic states. When you combine these, the result is a lot of states that can be transforming on picosecond to nanosecond scales. The c-termini transitions are on a nanosecond scale.
For these two states, we have devised a simulation that shows how they oscillate and eventually collapse, and how they couple to ionic states. We have discovered through simulation that states of c-termini may either be triggered or coupled to ions along the length of microtubules. All of this is now being integrated with signal transduction and with action potential.
Action potentials involve movement of many ions across the membranes. These may trigger state changes in microtubules as well. Putting it all together, I did a back end of the envelope computation. There are four c-termini states per dimer because we have two states per monomer.
There could be at least four states per electron inside the tubulin dimer, as they hop between two locations. There could be at least two computational changes due to the GTP hydrolysis. Thus there are 4 x 4 x 2, which is 32 states per dimer; thirteen dimers per ring; and 1,250 rings per midsize microtubule.
Footnote
1. MAP – To locate a gene or DNA sequence in a specific region of a chromosome in relation to known genes or DNA sequences. Stedman’s. The American Heritage Medical dic·tion·ar·y. Boston, New York: Houghton Mifflin Company, 2004.
(back to top)